High kinetic processing – „MechanoRecycling“
Making more with less – describes technological increased efficiency of process and product and includes ultimate recycling as a matter of course. Every waste consists of material and therefore of recyclable material. Common industrial processes are often associated with high energy and material costs to add value. A particularly resource-saving method is the mechanical recycling by high kinetic mechanochemical processing. Successful testing has been performed e.g. for the recovery of zinc from electric arc furnace dusts, the removal of flame retardants from polystyrene and the utilization of desert sand as construction sand.
Introduction
In a sustainable economy, industry and trade are engaged in the question of the life cycle of products and goods. In addition to the manufacturing processes, recycling plays an important role for any circular economy. Depending on the material, product or good, different recycling processes have to be developed and used. The way of recycling can turn out very differently. For example, poorly soluble materials are usually chemically or physically digested before further use, or heterogeneous materials must be broken down to their components in previous steps. The fact that this cannot always be done easily is shown in the composites, which often consist of more than two polymeric or other components.
Milling processes
Milling with drum or planetary ball mills is well-known in the field of mechanical process engineering [1]. Here, materials are mainly comminuted and partly deformed or mechanochemically transformed. Since the maximum relative velocities of the grinding media of these methods are in the range of 0 and 5.5 m/s, the resulting energy in the process must be classified to be in a low-kinetic range (Fig. 1) [2]. The velocity of the grinding media describes the energy that is transferred into the material to be ground, namely quadratic according to the equation of the kinetic energy [3]:
A distinctive feature of the different grinding processes is the way of movement of the grinding media. In the case of the drum, the planetary and the agitator ball mill, the grinding media usually move over each other in a rolling or falling manner due to the movement of the grinding vessel or the agitator. The resulting effects are categorized as cascade and cataract effects, respectively (Fig. 2) [4].
Due to the effects described, the material is mainly affected by shear and friction stresses, which contri-butes to the fact that the mostly powdery materials are comminuted similar to a grinding process with a physical increase of the surface area. With appropriate milling times and energy input, mechanochemical processes can also take place here. However, due to friction, these are usually associated with an increased degree of contamination of the material to be ground, since the grinding media can also be abraded. Contrary to these effects are collisions of the grinding media as they occur with high kinetic processing (HKP).
High kinetic processing
In comparison to conventional grinding processes, HKP has a significantly higher relative velocity of the grinding media. Likewise, less shear and friction effects are achieved due to the type of ball movement, but a collision effect is achieved which causes a selective transfer from the grinding media into the material. It can be therefore concluded that the energy that is transferred into the material by HKP is by potencies higher that that with conventional milling techniques.
At the same time, most processes run in one step without solvents (dry milling). In principle, wet milling is also possible, but in most applications it is neither necessary nor desirable. HKP can be used in many ways, with the various fields of application serving as the basis for subdividing the HKP into three different process categories: Mechanical Alloying (MA), High Energy Milling (HEM) and Reactive Milling (RM) [2]. All these categories have in common that the material to be processed is influenced and modified by formation and modification of nanostructures. Processes can also be carried out under vacuum or inert gas, including system loading [5]. This is especially important as fine dusts and metals are often used, which tend to be pyrophorous due to their high surface area and therefore have to be handled under exclusion of oxygen.
In MA, the energy of the grinding media is transferred into the material at a high yield. As a result, new alloys can be formed in a process of breaking, welding and deformation (deformation-fracture-welding), which would not be formed by conventional thermal syntheses. One example of a material made by MA is e.g. the recently relaunched PM2000, a high-temperature powder material that is consolidated into semi-finished and finished parts in subsequent steps [6].
In HEM, particles are broken by collisions, materials are refined and particles are deformed. For example, by HEM in a simple one step process zinc flake pigments are produces from zinc dust, which can have similar or even improved properties compared to commercially available zinc flakes. Nanostructures can be formed and new elements can be added in situ [7].
Reactive milling is the segment that also deals with the formation of new materials. Here, chemical reactions are carried out by the energy introduced, similar to a chemical reactor. In contrast, however, in a one-pot reaction without use of solvents as a carrier [8].
Based on the process variants described, different recycling strategies were developed in projects and tested in the field.
MechanoReSt – alternative recycling by mechanochemical treatment
In steel mills so-called EAF dust is formed by the electric arc furnace process (Fig. 3). This can be equated with a slag and contains besides common slag components resources like zinc and iron. These, especially zinc, are currently being extracted from the slag residue by digestion processes.
The process for the preparation of EAF dusts and the recovery of zinc from them is the so-called Waelz-process. Here a metallurgical process in a rotary kiln is carried out. In general, the Waelz-process is based on the enrichment of valuable substances in an intermediate metal phase, from which they are subsequently recovered after counterflow evaporation and re-oxidation. The process takes between 4 and 12 hours depending on the speed of rotation and the length of the rotary kiln. This process is energy-intensive and can only be regarded as economical from a zinc content of 20 %. The upper limit is 50 % zinc due to the otherwise excessive heat required. Here, a new energy-efficient and environmentally friendly process for recovery has to be developed.
This approach was pursued in a joint project sponsored by the German Federal Environmental Foundation (DBU) between Zoz GmbH and the Fraunhofer Institution for Materials Recycling and Resource Strategies IWKS, the “MechanoReSt” project (alternative recycling of environmentally critical metals from EAF dust by mechanochemical treatment). Deutsche Edelstahlwerke were associated partners (Grant No Az 33882/01). In addition to the recovery of zinc from EAF dusts, the aim of the project was to extract heavy metals as completely as possible from the residual mineral fraction. By this elimination of pollutants the fraction can then be used without problem, without the risk of pollution of groundwater or soil through elution processes. This is also an advantage over the Waelz-process, since the remaining heavy metals, even if immobilised, remain in the slag. The process developed here can also be used to digest poorly soluble compounds such as franklinite (ZnFe2O4) and to recover the incorporated zinc [9; 10; 11].
As part of the project, the EAF dusts were pre-treated mechanochemically and then leach with hydrochloric acid. The resulting suspension was filtered and the filtrate was cleaned in several steps by a wet chemical process by precipitation with appropriate substances ad freed from heavy metals (e.g. lead, cadmium, iron). The pure zinc was recovered from the last fraction by means of electrolysis. This shows the capability of mechanochemistry. Due to the high energy input into the material, it is also possible to digest even poorly soluble substances that would otherwise have to be processed in energy- or material-intensive digestion processes. Here, the processes for digestion are comparatively short (20 min process time) compared to the thermal processes mentioned above.
Recycling of polystyrene
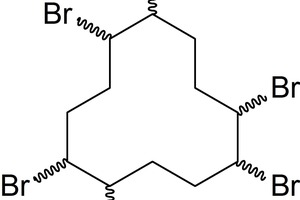
Every year, 40 000 t of old insulation materials have to be disposed. Due to the scarce capacity of appropriately certified waste incineration plants and the high demand, disposal is cost-intensive. A look at the insulation market shows that it is based on 34 % polystyrene products (EPS: expanded polystyrene, XPS: extruded polystyrene), which corresponds to 6392 t (Germany, 2012) [12]. In comparison, only 5 % of the market is based on polyurethane. The recycling and disposal of polystyrene products is an environmental problem because they contain toxic flame retardants. Hexabromocyclododecane (HBCD) is one of them and was used until the end of 2014 (Fig. 5).
This is homogeneously dispersed in the polymer matrix and not chemically bound. Depending on the storage time of the old polystyrene, it is visible as a brownish colour on the surface due to bromine (Fig. 6).
In 2008 HBCD was included in the SVHC list “Substances of very high concern” according to REACH and was subject to approval from then on. As a result, polystyrenes were considered “hazardous waste” according to the Waste Catalogue Ordinance (German: Abfallverzeichnis-Verordnung: AVV) and the POP-regulation, because they contain persistent organic substances (POP), HBCD has been classified as a POP since 2013 due to its toxic properties for reproduction. Consequently, they had to be disposed specially [13]. Pyrolysis of the materials causes the formation of even more toxic compounds, and extraction with solvents would create other problems. This regulation caused the disposal costs rise in some cases up to 7000 €/t.
After a Federal Council decision of 16.12.2016, the provisions of the AVV set two months earlier were overridden by a moratorium for one year, so that waste with HBCD did not have to be disposed separately as hazardous waste, since the AVV HBCD explicitly has been listed as an exception to the POP-regulation. This moratorium was declared a permanent derogation by the Federal Council on 07.07.2017, so that no special permission for the disposal of HBCD-containing PS waste was required [14]. Nevertheless, old polystyrene still contains HBCD and the change in the AVV thus prevents the necessary development towards resource-saving recycling. The problem has been levelled, but not eliminated.
A patent has been filed for a mechanochemical process with view to a resource-saving recycling process. Here HBCD is converted into non-toxic soluble compounds using HKP and quartz sand as tribomaterial. A comparable concept was also used and patented for the detoxification of dioxin (DE 102 61 204 A1) [15]. The process for recycling PS described here also produces, among other things, a black sand composite that could be used as construction sand. The conversion of HBCD into non-toxic soluble substances has also been successfully demonstrated analytically. Due to its conditions, this mechanochemical process is sustainable, energy-saving and of economic advantage, since mechanochemistry, in contrast to thermal recycling, requires less energy and, due to its short duration, the process can also be carried out more quickly.
Desert sand and micro plastic as construction material
Sand is currently considered a dwindling resource as construction material worldwide, although there are many deserts in which sand is virtually abundantly available. The problem is the sand grains themselves. Contrary to the comparable sea or river sand, these are round and not angular. As a result, the grains are excellent for hourglasses, but not for construction applications, since these would roll off from each other and the resulting concrete would lose the strength it gains from coarse grain. This means that approx. 95 % of the sands available worldwide cannot be used as construction materials. Every year, 40 billion tonnes of sand are exploited which is more than new erosion can create. The result is the destruction on entire ecosystems and the emergence of criminal, sometimes mafia-like structures that illegally mine sand on beaches and rivers. In Indonesia, for example, two dozen islands have already disappeared, and solutions to this problem are being sought worldwide [16; 17].
Another problem that is equally urgent to solve is the large quantities of microplastics that pollute the world’s oceans and whose disposal proves to be complex. Between 1950 and 2015, 6300 million t of plastic waste were generated and it is assumed that around 12,000 million t will be in the environment in 2050. Microplastics are currently produced in many areas of life and the discussion about their recycling is currently a focal topic in public life. First of all, of course, microplastics should simply be avoided. The problem with the released microplastics is how the quantities can be collected. If this succeeds, the question remains how it can be separated and recycled in an environmentally friendly manner [18]. HKP offers a simultaneous, possible solution to both global problems.
By simultaneously processing desert sand with/and microplastics with HKP, the sand is broken up so that it becomes edgy and loses its round shape. On the other hand, the sand transforms the microplastics into a kind of adhesive sand composite, which is then available for construction applications. The production of such a composite could be demonstrated in initial experiments. If industrial recycling succeeds here, the costs for construction sand would decrease significantly, along with the disposal costs for microplastics. Starting from an 1:1 composite microplastics/desert sand, e.g. with a Simoloyer® CM900, 5022 t/a of microplastics can be recycled in this way. Socially speaking, making desert sand usable would help conserve ecosystems and eliminate the aforementioned crime.
Conclusion
HKP technology represents an efficient technology, which, in addition to the advantages in the field of material production (new alloys, fine chemicals and pharmaceuticals, pigments), also offers many possibilities in the field of recycling and reprocessing. The energy transferred into the recycling materials is followed by chemical and mechanical conversions, but the recycling options are far from exhausted. Proven in tests, the method itself offers a high degree of implementation and purity. Due to the possibility on inert processing, materials can also be implemented that can only be recycled with great effort in a reactor process. In addition, the completely dry process eliminates the reprocessing of solvents, such as those used in a reactor process. Both underline technology sustainability.


![1 Comparison of the maximum relative velocity of the grinding media in different grinding processes [2]](https://www.recovery-worldwide.com/imgs/1/5/7/5/4/8/6/tok_f524a339b76720868d644b6665c22407/w300_h200_x600_y160_01_Fig._1_english-cbc07e8d3f92d366.jpeg)
![2 a) Cascade effect: balls roll over each other, b) cataract effect: balls lift off at a certain speed of rotation an hit again within the ball filling [4]](https://www.recovery-worldwide.com/imgs/1/5/7/5/4/8/6/tok_05406eeaac4f99e5878ef40f729e6a7c/w300_h200_x297_y421_02_Kaskaden-7f4c91419f1a3a21.jpeg)