Recycling chain for spent lithium ion batteries
The recycling of used lithium ion batteries is playing an increasingly vital role in respect of environmental policy, economic, geostrategic and health aspects. For the clear characterization and differentiation of the industrial recycling technologies and those in development, the model of the recycling chain is applied for used lithium ion batteries. In this context, the different treatment steps are presented and classified in process stages and main process groups.
1 Introduction
Lithium ion battery technology is currently one of the most important mobile energy storage units for electric and electronic applications. Thanks to their favourable properties, lithium batteries are the fastest-growing type of battery with the best prospects of success on the international market. Their recycling has vital importance as used lithium ion batteries sometimes contain not only geographically unevenly distributed, rare and valuable materials, but also cause large quantities of metal-containing waste. Here the motivation to recycle lithium ion batteries is based primarily on national or supranational legislation (Battery Act; Directive 2006/66 EC) and the intrinsic valuable content of the used batteries.
For waste treatment, up to now mainly robust pyrometallurgical recycling technologies have been used, which, however, exhibit only low recycling efficiency while requiring a high energy input at the same time [1]. Research is therefore being conducted internationally into the new and further development of alternative mechanical processes in combination with primarily hydrometallurgical recycling technologies. With the increasing importance of the process steps upstream of the metallurgical treatment, in this article the individual treatment steps for the recycling of lithium ion battery waste along the generic process chain for waste are theoretically grouped and, building on this, the methods and technologies used for treatment are presented.
2 Process stages and main process groups of the recycling chain for lithium ion batteries
Lithium ion batteries are recycled in defined process stages mostly with characteristic process engineering as well as subordinated process groups. Generally, the recycling chain consists of four process stages, each with two main process groups (Fig. 1) [2; 3]. Mixed used batteries are sorted during preparation either by battery type or active materials. Within the downstream process stages pretreatment and processing, the first waste-derived materials are produced and fed to established recycling processes. In the last process stage metallurgy, especially the treatment of the concentrates of active materials is examined. For completeness, however, it also includes the processes for material or raw material recovery of the organic solvents as well as the metallic and synthetic constituents, which, however, are not examined here further. The general objective of the respective main process groups is discussed in detail by Martens and Goldmann [2]. In the following, these are explained for used lithium ion batteries.
3 Preparation
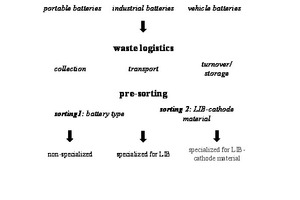
The main process groups in the first process stage are the waste disposal logistics and presorting (Fig. 2).
3.1 Waste management logistics
Used lithium ion batteries are currently available mainly as portable batteries. Depending on the legislation of the respective state, they are collected by means of different collection systems, transported to the next processing unit and transferred [4]. Vehicle batteries have to be accepted back by distributors (according to the Battery Act). In general, there is still great potential for collection of used lithium ion batteries, as for example collection quotas of 45 % for portable batteries in Germany are often not achieved elsewhere in the world [5; 6], many portable batteries are subject to hoarding [7] or find their way via the household waste to incineration plants or to landfills [8; 9; 10]. In addition, waste electrical and electronic equipment, which contains lithium ion batteries, are exported illegally to African and Asian countries. Collection systems for industry batteries for electromobility are either available from the vehicle manufacturers in certain regions in the world or currently developed in cooperation with local recyclers [8; 11].
As a consequence of the considerable hazard potential, the waste management logistics requires for used lithium ion batteries compliance with specific measures for protection against short circuits and leakage of the electrolyte [12]. Classification as hazardous material necessitates the use of special transport containers, warning signs, packaging and compliance with defined packing densities [12; 13].
3.2 Presorting
Used batteries of any origin are generally available as mixed batteries comprising different battery types or lithium ion batteries with different composition [7; 8; 14]. For this reason it is, however, not possible to recover all constituents from the mix in one single recycling process [15]. For recycling technologies specialized in lithium ion batteries, presorting by battery type is therefore necessary.
The sorting technologies used for presorting are classified under picking [8; 13; 15] so that recycling technologies building on these can in principle achieve high recycling quotas [5]. Moreover, for one lithium ion battery type, sorting can be performed according to the different cathode coatings. However, this still requires a previous breakdown of the battery cells to the functional unit and analysis of the active materials.
Most recycling technologies currently used work without presorting. The changing composition of the fed mixed material systems leads to a low recycling quota [5].
4 Pretreatment
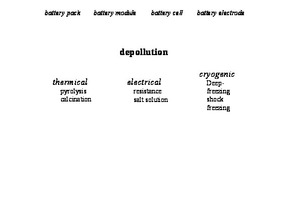
Used lithium ion batteries are then dismantled down to a defined depth and critical constituents or states are deactivated thermally, electrically or cryogenically (Fig. 3). Here, depending on size or the original purpose of the battery system, the batteries are pretreated both linearly or also iteratively within the process stage and main process groups.
4.1 Disassembly
With the increasing market penetration of electric vehicles and the associated increase in the volume of batteries returned, disassembly is expedient and in some cases imperative. On account of the complex structure of the used batteries, disassembly is a time-intensive process step. Access to the battery modules or cells is, however, essential for some methods of the removal of harmful substances. Disassembly from system to module or cell level generates on account of the high content of peripheral components products of metals, plastics and electronic components, which are fed to established recycling routes (Fig. 4) [1]. Furthermore, functional components or assemblies can be recovered for second-life applications.
Established are manual, semi-automatic (hybrid) and fully automatic disassembly concepts [16]. Purely manual disassembly is difficult to realize because of economic and safety aspects [14]. Hybrid concepts try to overcome this with a combination of manual activities in association with robots [17]. The implementation of standardized and automated disassembly by means of industrial robots is the subject of controversial discussion on account of the diverse design details of the wide variety of battery designs already available and also the rapid technical further development of batteries [12; 14; 17; 18]. Currently, the battery system is disassembled by hand to the required level [9; 14; 18]. Here, for work safety reasons, it must be ensured that at least the voltage is reduced to below 60 volt DC voltage as a precaution [14].
4.2 Removal of harmful substances
The hazard potential of the harmful substances for used lithium ion batteries can be summarized in electric, chemical and thermal categories [19]. Because the individual categories interact with each other, these must always be examined holistically within the framework of recycling. Accordingly, the removal of harmful substances is of crucial importance. Depending on the recycling process and target materials, this process is realized with different methods.
Discharge is one method that deactivates the residual chemical energy contained in the battery cells [11; 20]. Various methods for electric discharge were the subject of investigation in the LithoRec II research project [16; 21; 22]. In addition, batteries are discharged in special saline solutions [19; 23; 24; 25], in some cases with added iron particles [26], as well as in powders of conductor films or graphite [24]. Unwanted secondary reactions that lead to the corrosion of electric contacts or casing components, resulting leakage of the latter, are only rarely discussed [11; 22; 24]. Overall, the discharge leads primarily in respect of high-performance batteries from electric vehicles to high expense and cannot be applied to batteries with internal damage.
One method that avoids exothermal reactions during liberation comminution is cryogenic treatment. At temperatures around -200 °C, ion mobility decreases considerably [19]. However the chemical reactions taking place at staggered intervals after liberation.
Thermal processes like pyrolysis or calcination are another method. These deactivate the highly flammable electrolyte components and partially decompose components like the separator foil as well as the binder of the electrode coatings. As a result, the coating of the current conductor foils is removed, which is selectively utilized in a series of processes [14].
5 Processing
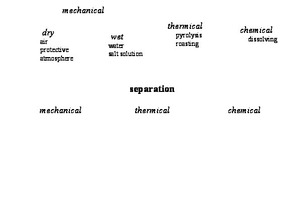
The third process stage in the recycling of lithium ion batteries is processing with the main process groups dissipation and separation (Fig. 5). The goal of this process stage is the liberation of individual materials based on the break-up of the casing and the functional unit as well as the separation of the individual components.
With a combination of mechanical macroprocesses like comminution, classifying and sorting, waste-derived raw materials are produced for the purpose of material recycling and raw material recycling or waste disposal are prepared [4]. In addition, thermal, chemical and other mechanical macroprocesses are used for specific tasks such as electrolyte separation, coating removal or compaction of comminuted and enriched materials [21; 27].
5.1 Dissipation
The mechanical liberation of battery modules and cells is realized by mainly by means of comminution with shearing, cutting and tearing stresses. Machines with impact systems tend to compaction and material inclusions [28]. The comminution of modules generally takes place in two stages with primary and liberation comminution [10; 29], in rarer case in a single stage [14; 22]. In addition, the electrohydraulic comminution of lithium ion batteries or components is investigated. An important aspect is the protection of the tools and process chambers used against the corroding effect of the electrolytes and electrode coatings [11].
This taken into consideration, depending on the type of pretreatment and removal of harmful substances, safety precautions have to be taken. These are oriented, for instance, to the medium that fills the process chamber, as strongly exothermal decomposition reactions up to explosions can occur [25]. Ideally, every cell is completely discharged for mechanical liberation [3; 11; 27]. If the hazard potential emanating from the volatile electrolyte components is reduced by means of thermal pretreatment, it is also possible to comminute in ambient air. If the batteries are not sufficiently discharged in advance, the batteries should be comminuted either in protective gas [25], like carbon dioxide [28], nitrogen [16], argon [28] or helium [19], or in liquid media, such as water or saline solutions [28]. In water, on account of undesirable secondary reactions of the electrolyte with the process medium (for example, formation of hydrofluoric acid), other safety precautions must be taken.
5.2 Separation
The separation of the components and materials contained is performed mainly by means of mechanical sorting. As separation processes, magnetic and eddy current separation, screening, gravity separation in flowing media [11] and air separating tables [19; 28] as well as flotation are used [25; 28]. Methods for electrolyte separation have been investigated, for example, by Kwade and Diekmann [21].
While the combination of sorting steps is very process-specific, the materials in almost all recycling processes are concentrated in products such as casing material (Fig. 6), plastics, electrode foils (Fig. 7) and coating [21; 27; 30]. In some cases, after liberation comminution, the electrode foils still contain coating materials that are separated by means of other mechanical [1; 27] or thermal stresses as well as chemical treatment [25; 31].
For sorting of the active materials themselves, flotation [32] or multistage magnetic separation [33] with corresponding preparation steps are the subject of research. Although further mechanical processing of the active materials is the preferred variant, successful separation is only possible to a limited extent on account of the small particle sizes, the low degree of liberation and the hardly existing differences in the material properties.
5.3 Metallurgy
In the last process stage in recycling of lithium ion batteries, the prepared and pretreated used batteries or their process constituents (Fig. 8), especially the metals cobalt, manganese, nickel and iron in the cathode coating, are sent to extractive metallurgy for the production of basic materials. As the main process groups extraction and recovery are performed in combination, refining is organized according to the hydro- and pyrometallurgical processes applied (Fig. 9).
5.3.1 Pyrometallurgy
Depending on the composition of the feed material and the process-specific parameters, the metals cobalt, nickel, iron and copper are collected as alloy in a molten metal phase. This can be either a finished product like, for example alloyed steel, or an intermediate product, which is rarely further refined in a pyrometallurgical process, but in most cases in hydrometallurgical processes. In the pyrometallurgical processes, lithium, manganese and aluminium find their way into the furnace slag and are currently used as filling material in road construction [5; 30] or landfilled [10]. Technical possibilities for recovery do exist in principle [10], but are not applied at present for economic reasons [6; 23; 28]. Moreover, organic components are pyrolyzed. The graphite content of the material to be smelted influences in addition slag formation and the success of the overall process. In addition, pollutants such as carbon dioxide, dioxins and furans are formed [25; 31].
Overall, low capacities, a high energy consumption and a limited recycling efficiency clearly show the disadvantage of pyrometallurgical processes [31]. In contrast to this is the robustness of this technology, which requires only limited pretreatment and conditioning of the feed material.
5.3.2 Hydrometallurgy
Hydrometallurgical processing of the metals from the active materials generally takes place after the three macroprocesses dissolution, concentration and cleaning as well as recovery of metal alloys [34]. As solvents, various organic and inorganic acids have been investigated [31; 34]. Besides these, leaching methods for the chemical conversion of metals by means of bioorganisms are the subject of research.
In respect of material preparation, the hydrometallurgical processes require, besides manual disassembly, comminution and separation. The manual disassembly of batteries to electrode level supplies high-purity starting materials and the required particle sizes for further hydrometallurgical processing. For industrial realization, manual removal of the valuable cathode materials, however, does not appear expedient owing to economic and work safety considerations. If the electrode coatings are removed by means of mechanical stress, in many cases copper and aluminium particles from the current-conducting foils find their way as impurities into the coating fraction [27]. Other impurities are residues of the organic solvents.
Generally, strong organic acids and expensive additives are necessary. Also considerable quantities of liquid waste are produced. On the other hand, especially the metals from the cathode coating can be selectively and energy efficiently recovered [19].
6 Summary
The generic process chain for waste of Martens and Goldmann [2] offers the possibility to classify the different treatment steps for material recycling of used lithium ion batteries in four process stages and the associated main process groups. With this, the different recycling technologies can be clearly classified, differentiated and process specifications can be addressed.
The focus of research has so far been on the process stage metallurgy with pyro- and increasingly hydrometallurgical processes. On laboratory scale, the starting materials relevant for this (cathode active materials) are usually recovered by means of manual disassembly of the battery systems with subsequent breakdown of the battery cells to electrode level. In industrial plants, mechanical liberation comminution with subsequent sorting is necessary, which significantly influences metallurgical further processing.
The production of secondary raw materials or materials derived from used batteries is technically possible in principle. However, the current cost of material recycling is high compared with the production of primary raw materials and materials. Crucial for material recycling remains the collection of used batteries and the profitable sale of the waste-derived raw materials produced [35].


![Recycling chain with process stages and main process groups for used lithium ion batteries [3]](https://www.recovery-worldwide.com/imgs/1/5/1/6/4/0/2/tok_42ec397d80b34fe7d85eb5907b544ff7/w300_h200_x297_y421_01_battery_Werner_en-e2e27bf53c428767.jpeg)